NutraVault
Gastro-resistant enteric encapsulation for oral delivery of nutraceuticals, prebiotics, probiotics, enzyme therapeutics and dietary supplements
The commercial need
The market for nutraceuticals, prebiotics, probiotics, enzyme therapies, and dietary supplements are growing strongly and there is a solid commercial need for an effective gastro-resistant enteric encapsulation made from safe bio-inactive materials.
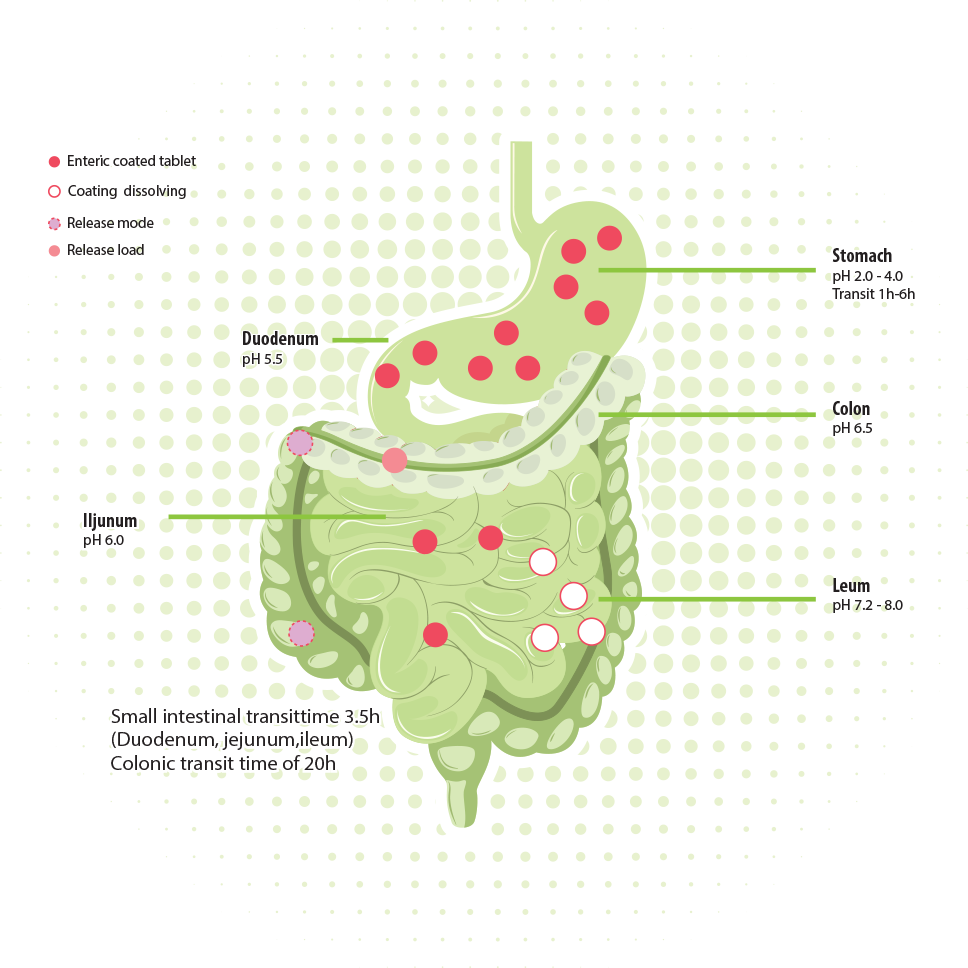
Nutraceuticals are primarily delivered orally. Many nutraceuticals are vulnerable to low pH and protease digestion in the stomach. They need a gastro-resistant encapsulation to protect them to pass the stomach without reducing or losing their effect.
The current technologies for oral delivery cannot deliver safe, reliable, and convenient delivery to the colon. The series of NutraVault encapsulations protect during stomach passage and ensure targeted release either in the small intestine or in the colon.
Encapsulations with safe bio-inactive materials
Also, hypromellose derivatives and polymethacrylates have gained attention as a protective layer for nutraceuticals. However, according to regulatory law, nutraceutical encapsulations must be approved as a food additive, and due to their synthetic nature and potential bioactivity EMA and FDA only approve intake in limited amounts and not for use in “over the counter” products like nutraceuticals.
Oral prebiotic/probiotic administration
Companies like EnteroBiotix, Microbiotica, MaatPharma, Sniprbiome, Eligo, Caelus Health and Chainbiotech are examples of biotech companies that have microbiome and microbiota therapeutics in development. In general, there are high expectations to these types of products.
The GI survival of Live Microbiotics is highly variable. Is it highly important to use the “appropriate vehicle for the bacteria to reach its site of action “.
Some bacteria strains (i.e. lactobacillus acidophilus, bifidobacterium, streptococcus mutants, etc.) partially resist the acidic environment of the stomach and the high bile salt conditions of the intestine, but others (i.e. lactobacillus delbrueckii, streptococcus thermophiles, escherichia coli Nissle 1917, etc.) do not survive the harsh conditions in the stomach. The survival of these probiotics through the stomach could benefit from protection by NutraVault.
Lactobacillus delbrueckii and streptococcus thermophilus are marketed for the improvement of digestive health. Escherichia coli Nissle 1917 is also marketed for its beneficial effects as a probiotic and is tested for its effect on the indications gastroenteritis, laryngitis, otitis media and irritable bowel syndrome.
Further, Lactobacillus paracasei, has effect on gastroenteritis, rhinitis, otitis, laryngitis, and tracheitis. However, these probiotics could benefit from protection by NutraVault by getting targeted delivery of a better-defined amount of the probiotic to the intestine.
